How to Calculate Acid Neutralizing Capacity
We can try the Kjeldahl method with a sample for the test nitrogen content a Kjeldahl flask a heat resistant long-necked flask made of borosilicate glass used in experiment labs a condenser and some basic laboratory equipments. Acid-Neutralizing Capacity Certainly this test is applicable only to measure the acid-neutralizing capacity of an antacid tablet.

Acid Neutralizing Capacity Of An Antacid
Adding 76 oz or 475 lbs of boric acid per 10000 gallons of water will provide 10 ppm of borate.

. Procedures of Kjeldahl method. It is often sold in nurseries and garden stores as a bug killer. Boric acid is a weak acid and has a pH of 38-48.
Strong-acid resins exhibit only strong-acid functionality. Result 08 FM M 09 FC C. NLT 5 mEq of acid is consumed by the minimum single dose recommended in the labeling and the number of mEq calculated by the formula 2.
Boric acid is sold at home centers hardware and gardening stores. This means that the total capacity is not capable of salt splitting but it is capable of neutralizing acid. As a result of manufacture or composition strong-base resins can exhibit a degree of weak-base function.
The Kjeldahl method is performed in three steps namely digestion distillation and titration. The chemical formula is BOH3 or H3BO3 same thing.

Measurement Of Acid Neutralizing Capacity
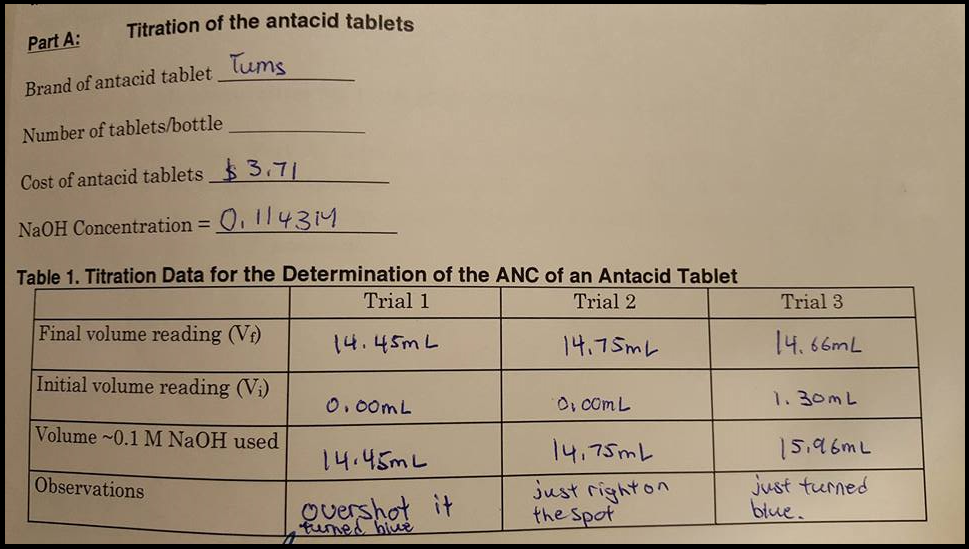
Acid Neutralizing Capacity Of Antacid Tablets Need Chegg Com
No comments for "How to Calculate Acid Neutralizing Capacity"
Post a Comment